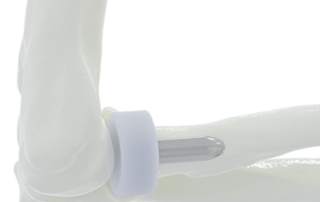
News
BioPoly® Radial Head System Receives FDA Clearance
FORT WAYNE, IN – BioPoly LLC announced today that the FDA has granted clearance on their new BioPoly® Radial Head System for the elbow. This implant is manufactured from BioPoly’s proprietary material and is the only synthetic cartilage Radial Head on the market. With this FDA clearance, BioPoly has taken significant steps toward achieving its strategy of expansion of its orthopedic, resurfacing implants in the US. The team has been actively developing multiple implant systems for many years as we worked toward gaining FDA clearance in the US market. Dr. Herb Schwartz, BioPoly’s Chairman and CTO, said, “We now have [...]
BioPoly® Announces Publication of 5-Year Clinical Study Results with the BioPoly Knee Partial Resurfacing System
FORT WAYNE, IN – BioPoly LLC announced today the publication of 5-year clinical study results on their BioPoly Knee Partial Resurfacing System in the Journal of Bone and Joint Surgery (JBJS). This follows the publication of their interim 2-year report in JBJS in 2017. See the publication here: https://tinyurl.com/BioPolyKnee “We’re proud of the results of our clinical study with our knee system in Europe and are encouraged with the continued positive clinical outcomes”, says Ryan Schlotterback, President & CEO of BioPoly. “We believe these results continue to demonstrate the value and clinical efficacy of our BioPoly technology in the exciting [...]
BioPoly® Announces Distribution Agreement with Veteran’s Health Medical Supply
FORT WAYNE, IN – BioPoly LLC announced today that they have entered into an agreement with Veteran’s Health Medical Supply (VHMS) for distribution of its products. VHMS will be the exclusive distributor of BioPoly products to U.S. government health care agencies, including VA health facilities. “The health and lives of our veterans is something we feel honored to be able to positively impact. We’re pleased that through this agreement we’ll be able to supply orthopedic solutions that provide pain relief and return to function for our veterans”, says Ryan Schlotterback, President & CEO of BioPoly. “It’s been a pleasure getting [...]
BioPoly® Announces FDA Breakthrough Device Designation of the BioPoly Knee System
FORT WAYNE, IN – BioPoly LLC announced today the FDA has granted the company a Breakthrough Device designation for its BioPoly Knee System. The company plans to execute on their FDA-approved study with the BioPoly Knee System in the US this year. The BioPoly Knee System utilizes the company’s proprietary BioPoly material which functions as synthetic cartilage. “This is a big milestone and achievement for the company and BioPoly team”, says Ryan Schlotterback, President & CEO of BioPoly. “We’re excited about the Breakthrough Device designation and the potential to accelerate our BioPoly Knee System into the US market. We have [...]
BioPoly® Appoints new Vice President of Sales and Marketing
FORT WAYNE, IN – BioPoly LLC announced today that Justin J. Kaler has joined the executive management team as the Vice President of Sales and Marketing. An experienced sales and marketing leader, Justin will be assuming responsibilities of this role and focusing efforts on commercialization of its proprietary BioPoly material technology in the United States. Mr. Kaler comes to BioPoly with more than 14 years in the orthopedic industry with experience in various leadership roles in marketing & sales at Biomet, Stryker, Zimmer Biomet and most recently Medartis where he was Chief Commercial Officer. “I'm extremely grateful for the opportunity [...]